การพัฒนาและตรวจสอบความใช้ได้ของวิธีวิเคราะห์หาปริมาณแคนนาบิไดออล (CBD) ในตำรับยาครีม 1% ซีบีดี ด้วยเทคนิคโครมาโทกราฟีของเหลวสมรรถนะสูง
คำสำคัญ:
แคนนาบิไดออล (CBD), โครมาโทกราฟีของเหลวสมรรถนะสูง, ยาครีม CBD, การตรวจสอบความใช้ได้ของวิธีวิเคราะห์บทคัดย่อ
ความเป็นมา: โรงพยาบาลเจ้าพระยาอภัยภูเบศรมีการพัฒนาตำรับยาครีม 1.0% w/w แคนนาบิไดออล (CBD) สำหรับใช้ในผู้ป่วยโรคสะเก็ดเงินและโรคผิวหนังอักเสบ ดังนั้น จึงมีการพัฒนาวิธีวิเคราะห์หาปริมาณ CBD ที่มีความแม่นยำและน่าเชื่อถือเพื่อควบคุมคุณภาพผลิตภัณฑ์
วัตถุประสงค์: เพื่อพัฒนาและตรวจสอบความใช้ได้ของวิธีวิเคราะห์เชิงปริมาณ CBD ในตำรับยาครีม 1.0% w/w CBD ด้วยเทคนิคโครมาโทกราฟีของเหลวสมรรถนะสูง (HPLC)
วิธีการศึกษา: วิเคราะห์หาปริมาณ CBD ในตำรับยาครีม ด้วยเทคนิค HPLC โดยใช้คอลัมน์ NexLeaf CBX for Potency (C18) ที่อุณหภูมิ 35 องศาเซลเซียส ความยาวคลื่น 220 นาโนเมตร วัฏภาคเคลื่อนที่ประกอบด้วย 0.085% orthophosphoric acid ใน deionized water และ 0.085% orthophosphoric acid ใน acetonitrile ในอัตราส่วนที่เปลี่ยนแปลงระหว่างการวิเคราะห์ (gradient elution) อัตราการไหล 1.6 มิลลิลิตร/นาที การตรวจสอบความใช้ได้ของวิธีวิเคราะห์จะประเมินในหัวข้อ การทดสอบความเหมาะสมของระบบ (system suitability) ความจำเพาะเจาะจง (specificity) ความเป็นเส้นตรง (linearity) ช่วงความเข้มข้น (range) ความแม่นยำ (accuracy) และความเที่ยง (precision) ตามแนวทางของ ICH Q2(R1)
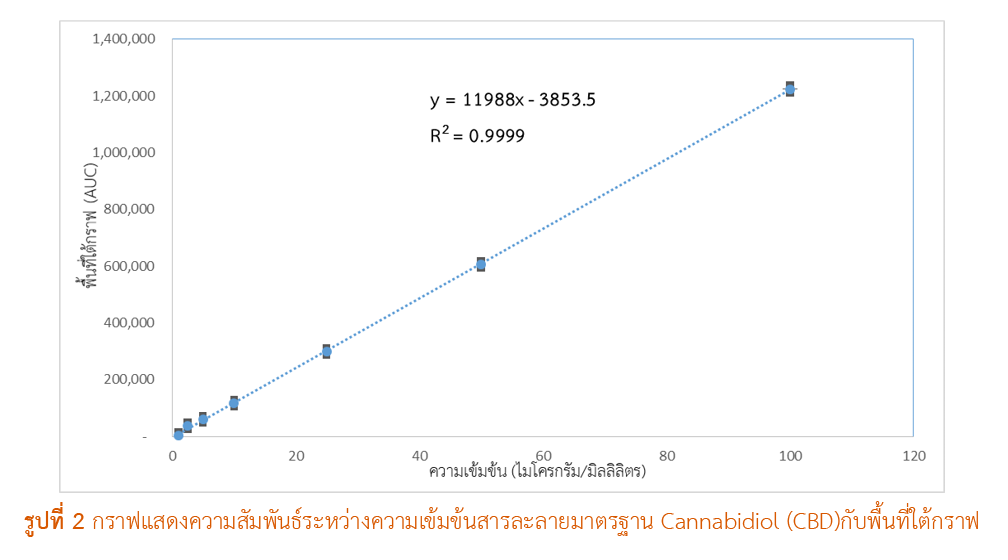
ผลการศึกษา: วิธีวิเคราะห์หาปริมาณที่พัฒนาขึ้นแสดงความจำเพาะเจาะจงในการแยก CBD โดยไม่มีสัญญาณรบกวนจากส่วนประกอบอื่นในสูตรตำรับยาครีม โดยมีความสัมพันธ์เชิงเส้นตรงในช่วงความเข้มข้น 1-100 ไมโครกรัม/มิลลิลิตร (R² = 0.9999) ความแม่นยำรายงานในค่าร้อยละการคืนกลับอยู่ในช่วง 98.5-101.2% และความเที่ยงรายงานในค่าเบี่ยงเบนมาตรฐานสัมพัทธ์ (%RSD) ต่ำกว่า 1% ทั้งในการทดสอบภายในวันเดียวกัน (intra-day precision) และระหว่างวัน (inter-day precision) เมื่อนำวิธีที่พัฒนาขึ้นกับการวิเคราะห์ตำรับยาครีม 1.0% CBD จำนวน 2 ตัวอย่าง พบว่าปริมาณ CBD ที่ตรวจวัดได้เท่ากับ 1.1023% และ 1.1918% w/w
สรุปผลการศึกษา: วิธีวิเคราะห์หาปริมาณ CBD ในตำรับยาครีม 1.0% CBD ด้วยเทคนิค HPLC ที่พัฒนาขึ้นเป็นไปตามข้อกำหนดมาตรฐานสากลสำหรับการวิเคราะห์สารออกฤทธิ์ในผลิตภัณฑ์ยา มีความแม่นยำ เที่ยงตรง และน่าเชื่อถือ สามารถนำไปใช้ในการควบคุมคุณภาพผลิตภัณฑ์ยาครีม CBD ได้
เอกสารอ้างอิง
Martinez Naya N, Kelly J, Corna G, Golino M, Abbate A, Toldo S. Molecular and cellular mechanisms of action of cannabidiol. Molecules. 2023;28(16):5980. doi: 10.3390/molecules28165980.
Baswan SM, Klosner AE, Glynn K, Rajgopal A, Malik K, Yim S, Stern N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin Cosmet Investig Dermatol. 2020;13:927-42. doi: 10.2147/CCID.S286411.
Puaratanaarunkon T, Sittisaksomjai S, Sivapornpan N, Pongcharoen P, Chakkavittumrong P, Ingkaninan K, et al. Topical cannabidiol-based treatment for psoriasis: a dual-centre randomized placebo-controlled study. J Eur Acad Dermatol Venereol. 2022;36(9):e718-20. doi: 10.1111/jdv.18215.
Sarma ND, Waye A, ElSohly MA, Brown PN, Elzinga S, Johnson HE, et al. Cannabis inflorescence for medical purposes: USP considerations for quality attributes. J Nat Prod. 2020;83(4):1334-51. doi: 10.1021/acs.jnatprod.9b01200.
ธนวัฒน์ ทองจีน, สรเพชร มาสุด, พีรธรรม เทียมเทียบรัตน์, สายัณห์ เรืองเขตร, ศักดิ์วิชัย อ่อนทอง, พิเชฐ บัญญัติ, และคณะ. การพัฒนาวิธีวิเคราะห์ปริมาณแคนนาบินอยด์ในใบกัญชาด้วยวิธี ultra high performance liquid chromatography. วารสารกรมวิทยาศาสตร์การแพทย์ [อินเทอร์เน็ต]. 2564 [สืบค้นเมื่อ 20 ก.พ. 2568];63(3):505-23. สืบค้นจาก: https://he02.tci-thaijo.org/index.php/dmsc/article/view/252742
Wilson WB, Abdul-Rahman M. Determination of 11 cannabinoids in hemp plant and oils by liquid chromatography and photodiode array detection. Chromatographia. 2022;85(1):115-25. doi: 10.1007/s10337-021-04114-y.
Mandrioli M, Tura M, Scotti S, Gallina Toschi T. Fast detection of 10 cannabinoids by RP-HPLC-UV method in Cannabis sativa L. Molecules. 2019;24(11):2113. doi: 10.3390/molecules24112113.
Schettino L, Prieto M, Benedé JL, Chisvert A, Salvador A. A rapid and sensitive method for the determination of cannabidiol in cosmetic products by liquid chromatography–tandem mass spectrometry. Cosmetics. 2021;8(2):30. doi: 10.3390/cosmetics8020030.
Galant N, Czarny J, Powierska-Czarny J, Piotrowska-Cyplik A. Development and validation of the LC–MS/MS method for determination of 130 natural and synthetic cannabinoids in cannabis oil. Molecules. 2022;27(23):8601. doi: 10.3390/molecules27238601.
กรมวิทยาศาสตร์การแพทย์. สำนักยาและวัตถุเสพติด. Thai Herbal Pharmacopoeia 2021 Supplement 2024 [อินเทอร์เน็ต]. นนทบุรี: สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข; 2567 [สืบค้นเมื่อ 10 ก.พ. 2568]. สืบค้นจาก: https://website.bdn.go.th/th/e-book/detail/nGM4ZtWewEb3QWewEb3Q
United States Pharmacopeia. General Chapter, <621> Chromatography [Internet]. Rockville (MD): The United States Pharmacopeial Convention; 2025 [cited 2025 Feb 13]. Available from: https://doi.usp.org/USPNF/USPNF_M99380_09_01.html
The International Council for Harmonization of Technical Requirements of Pharmaceuticals for Human Use (ICH). Validation of analytical procedures - Scientific guideline Q2(R1) [Internet]. Amsterdam: European Medicines Agency; 1995 [cited 2025 Jan 21]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r1-validation-analytical-procedures-text-methodology-step-5-first-version_en.pdf
นพวัฒน์ เพ็งคำศรี. การตรวจสอบความถูกต้องของวิธีวิเคราะห์ทางเภสัชกรรม [อินเทอร์เน็ต]. นนทบุรี: ศูนย์การศึกษาต่อเนื่องทางเภสัชศาสตร์ สภาเภสัชกรรม; 2560 [สืบค้นเมื่อ 10 ม.ค. 2567]. สืบค้นจาก: https://ccpe.pharmacycouncil.org/index.php?option=article_detail&subpage=article_detail&id=338
AOAC. Appendix F: Guidelines for standard method performance requirements [Internet]. Rockville (MD): AOAC International; 2016 [cited 2025 Mar 11]. Available from: https://www.aoac.org/resources/guidelines-for-standard-method-performance-requirements/
Kühn-Hebecker A. New quality standards for medical cannabis and CBD [Internet]. Heidelberg: Concept Heidelberg GmbH; 2025 [cited 2025 Jun 1]. Available from: https://www.gmp-journal.com/current-articles/details/new-quality-standards-for-medical-cannabis-and-cbd.html
ดาวน์โหลด
เผยแพร่แล้ว
วิธีการอ้างอิง
ฉบับ
บท
การอนุญาต
ลิขสิทธิ์ (c) 2025 กองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมคลินิกทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของกองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสารเภสัชกรรมคลินิกอย่างเป็นลายลักษณ์อักษร
กองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับกองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข และบุคลากรในกองฯ หรือ ชมรมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ



