Developing Guideline for Surveillance of Renal Complication from Tenofovir Disoproxil Fumarate (TDF) in HIV Infected Patients at Nakhon Phanom Hospital
Keywords:
guideline for surveillance, TDF, renal complication, HIVAbstract
Blackground: Tenofovir disoproxil fumarate (TDF) is an antiretroviral drug in the basic regimen recommended for the treatment of HIV infection due to effectiveness. The main adverse reaction is the occurrence of renal complications which Nakhon Phanom Hospital has no clear guideline for surveillance of this complication.
Objectives: To develop a guideline for surveillance of renal complication from TDF and to study the effect of using the guideline.
Methods: This action research consisted of 4 phases 1) preparing 2) action 3) prospective data collection 4) outcome monitoring. The sample were 418 HIV infected patients who started receiving TDF in drug formulations during 1 October 2019 to 30 September 2021. The study instrument was the guideline for surveillance of renal complication from TDF developed by multidisciplinary team. Data collecting instruments were the record form for adverse drug event, drug related problems and medication adherence. Data were analyzed using frequency, percentage and comparative data on the incidence of severe renal complications from TDF before and after using the guideline by Fisher’s Exact test.
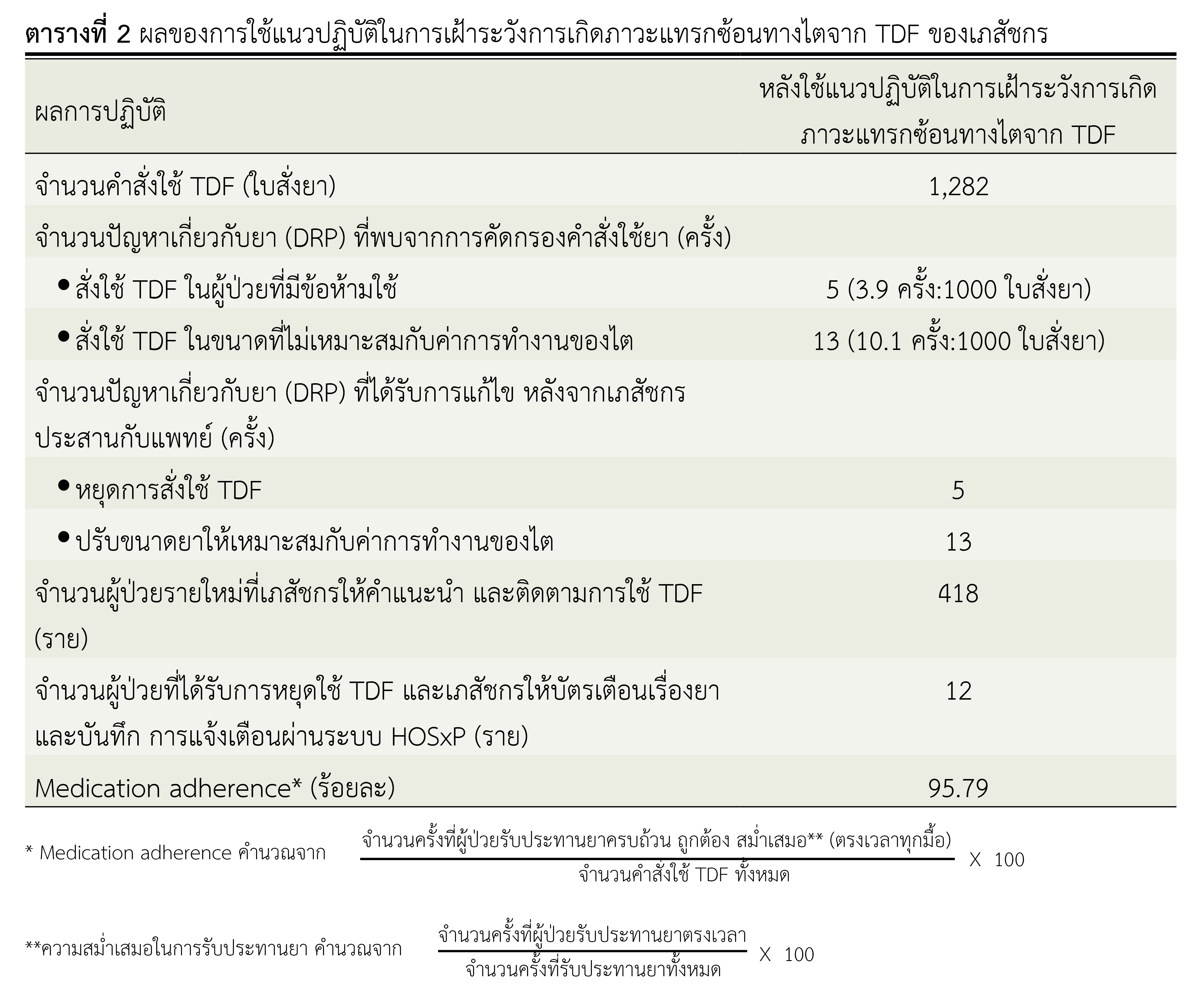
Results: A developed guideline for surveillance of renal complications from TDF in HIV infected patients consisted of guidelines for monitoring renal function, dosage adjustment according to renal function, drug discontinuation and drug counseling. The effect of using the guideline were as follows : 1) A total of 1,282 prescriptions were screened by pharmacist, 5 prescriptions were found with contraindication and 13 prescriptions were found with an inappropriate dose. After pharmacist recommendation physician agreed to discontinue the drug and adjust the dose in prescriptions all of them. 2) The incidence of severe renal complications from TDF before using guideline was 7 cases (1.93%), after using guideline was 1 case (0.24%). There has been significantly decreased (p<0.05).
Conclusion: The guideline for surveillance of renal complication from TDF that developed by multidisciplinary team could reduce the incidence of severe renal complications.
References
สุเมธ องค์วรรณดี และคณะ. แนวทางการตรวจรักษาและป้องกันการผู้ติดเชื้อเอชไอวีประเทศไทย ปี 2560. กรุงเทพมหานคร: สำนักโรคเอดส์ วัณโรค และโรคติดต่อทางเพศสัมพันธ์ กรมควบคุมโรค กระทรวงสาธารณสุข; 2560.
Ray A.S., Fordyce M.W., Hitchcock M.J. Tenofovir alafenmide: A novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016;125:63-70.
วิศิษฎ์ ตันหยง, พีรยศ ภมรศิลปะธรรม, ฉัตรชัย ฉิ่นไพศาล. ทีโนโฟเวียร์และพิษต่อไตระดับเซลล์. วารสารวิทยาศาสตร์บูรพา. 2560;22(2):248-59.
Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, Sanchez-Niño MD, Izquierdo MC, Poveda J, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. doi: 10.1155/2011/354908.
Irizarry-Alvarado JM, Dwyer JP, Brumble LM, AlvarezS, Mendez JC. Proximal tubular dysfunction associated with tenofovir and didanosine causing Fanconi syndrome and diabetes insipidus: A report of 3 cases. AIDS Read 2009;19(3):114-21.
Suzuki S, Nishijima T, Kawasaki Y, Kurosawa T, Mutoh Y, Kikuchi Y, et al. Effect of tenofovir disoproxil fumarate on incidence of chronic kidney disease and rate of estimated glomerular filtration rate decrement in HIV-1-infected treatment-naïve Asian patients: Results from 12-year observational cohort. AIDS Patient Care STDS. 2017;31(3):105-12.
Pichpattana M, Phiboonbanakit D, Trakulhun K, Supasyndh O. Incidence of tenofovir disoproxil fumarate induced proximal tubulopathy in HIV-infected patients. J Infect Dis Antimicrob Agents. 2016;33:33-55.
Bonjoch A, Echeverria P, Perez-Alvarez N, Puig J, Estany C, Clotet B, et al. High rate of reversibility of renal damage in a cohort of HIV-infected patients receiving tenofovir-containing antiretroviral therapy. Antiviral Res. 2012;96:65-9.
Izzedine H, Harris M, Perazella MA. The nephrotoxic effects of HAART. Nat Rev Nephrol. 2009;5(10):563-73.
Lyseng-Williamson KA, Reynolds NA, Plosker GL. Tenofovir disoproxil fumarate: A review of its use in the management of HIV infection. Drugs. 2005;65:413-32.
Asawatwong S. Tenofovir and risk of deficient renal function in HIV/AIDs patients at Krabi hospital. Krabi Medical Journal. 2018;1(1):35-43.
รชานนท์ หิรัญวงษ์, การเฝ้าระวังผลต่อไตจากการใช้ยาเทโนโฟเวียร์ ในผู้ป่วยติดเชื้อเอชไอวี โรงพยาบาลบางละมุง. ข่าวสารด้านยาและผลิตภัณฑ์สุขภาพ. 2555;15(4):117-22.
Hall AM, Hendry BM, Nitsch D, Connolly JO. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57(5):773-80. doi: 10.1053/j.ajkd.2011.01.022.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Health Administration Division, Office of the Permanent Secretary, Ministry of Public Health and The Society of Hospital Pharmacist, Ministry of Public Health

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
ข้อความภายในบทความที่ตีพิมพ์ในวารสารเภสัชกรรมคลินิกทั้งหมด รวมถึงรูปภาพประกอบ ตาราง เป็นลิขสิทธิ์ของกองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข การนำเนื้อหา ข้อความหรือข้อคิดเห็น รูปภาพ ตาราง ของบทความไปจัดพิมพ์เผยแพร่ในรูปแบบต่าง ๆ เพื่อใช้ประโยชน์ในเชิงพาณิชย์ ต้องได้รับอนุญาตจากกองบรรณาธิการวารสารเภสัชกรรมคลินิกอย่างเป็นลายลักษณ์อักษร
กองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข อนุญาตให้สามารถนำไฟล์บทความไปใช้ประโยชน์และเผยแพร่ต่อได้ โดยอยู่ภายใต้เงื่อนไขสัญญาอนุญาตครีเอทีฟคอมมอน (Creative Commons License: CC) โดย ต้องแสดงที่มาจากวารสาร – ไม่ใช้เพื่อการค้า – ห้ามแก้ไขดัดแปลง, Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0)
ข้อความที่ปรากฏในบทความในวารสารเป็นความคิดเห็นส่วนตัวของผู้เขียนแต่ละท่านไม่เกี่ยวข้องกับกองบริหารการสาธารณสุข สำนักงานปลัดกระทรวงสาธารณสุข และ ชมรมเภสัชกรโรงพยาบาลกระทรวงสาธารณสุข และบุคลากรในกองฯ หรือ ชมรมฯ แต่อย่างใด ความรับผิดชอบองค์ประกอบทั้งหมดของบทความแต่ละเรื่องเป็นของผู้เขียนแต่ละท่าน หากมีความผิดพลาดใด ๆ ผู้เขียนแต่ละท่านจะรับผิดชอบบทความของตนเอง ตลอดจนความรับผิดชอบด้านเนื้อหาและการตรวจร่างบทความเป็นของผู้เขียน ไม่เกี่ยวข้องกับกองบรรณาธิการ



